Health Canada approves Pfizer’s COVID-19 therapeutic
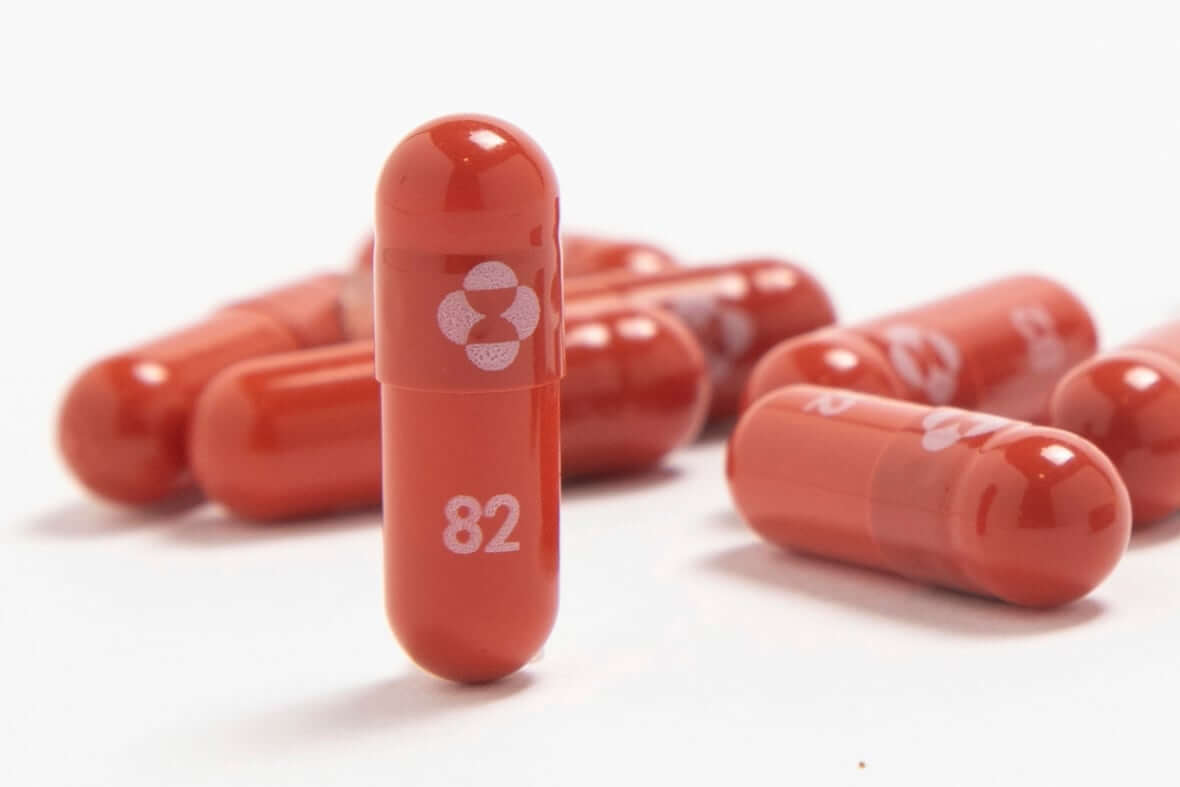
Health Canada has approved Pfizer’s COVID-19 therapeutic, paving the way for the distribution of this potentially lifesaving drug at a time when the country’s hospitals are overwhelmed.
Pfizer’s Paxlovid, which is an antiviral prescribed by a doctor and administered in pill form, is designed to help the body fight off the SARS-CoV-2 virus, reduce symptoms from an infection and shorten the period of illness.
The product has been hailed as a pandemic “game changer” by some doctors because it could reduce hospitalizations and deaths among COVID-19 patients. An effective pill that’s easy to self-administer at home could relieve some of the pressure on the health-care system and change the trajectory of the pandemic, experts say.
After months-long clinical trials, Pfizer reported in November that Paxlovid reduced the risk of hospitalization or death by an impressive 89 per cent compared to a placebo in non-hospitalized high-risk adults with COVID-19.
Canada has placed an order for an initial quantity of one million treatment courses. Some of that supply will start to arrive after Health Canada’s expected approval.






Redes Sociais - Comentários