Health Canada approves Pfizer-BioNTech’s COVID-19 vaccine for children

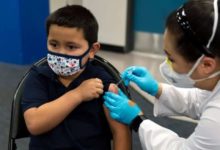
Health Canada has approved Pfizer-BioNTech’s vaccine for children aged five to 11, saying it’s more than 90 per cent effective against COVID-19 in younger kids.
“After a thorough and independent scientific review of the evidence, the department has determined that the benefits of this vaccine for children between five and 11 years of age outweigh the risks,” Health Canada wrote in a release Friday morning.
“This is the first COVID-19 vaccine authorized in Canada for use in this age group and marks a major milestone in Canada’s fight against COVID-19.”
Pfizer-BioNTech’s pediatric vaccine is delivered in doses one-third the size of those given to adults and kids 12 and older. Health Canada authorized a two-dose regimen to be administered three weeks apart.
The National Advisory Committee on Immunization (NACI), however, is recommending that the spacing between doses be increased to at least eight weeks, as evidence has been growing that a longer interval generates a more robust immune response. The longer spacing might also help to decrease even further the risk of the rare side effect of myocarditis (inflammation of the heart muscle), NACI said.
Health Canada said clinical trials showed the vaccine was 90.7 per cent effective at preventing COVID-19 in children five to 11 years of age and that no serious side effects were identified.
In a statement Friday, Pfizer said the new doses would be shipped “imminently.”
The provinces and territories are responsible for administering the vaccine. Ontario Health Minister Christine Elliott said the province’s booking system should be ready as early next week for parents to make appointments for their children.
Everyone benefits: doctor
Dr. Michelle Barton-Forbes is an associate professor at Western University’s Schulich School of Medicine and Dentistry in London Ont., who specializes in pediatric infectious disease. She said this could be a game-changer for Canada’s pandemic response.
“So ultimately, not only will we help to keep kids from acute COVID-19 and its consequences, we will keep kids healthy and we will also keep them happy as they are allowed to do in-class learning and extracurricular activities,” she said.
“This would also help reduce new adult cases in the community resulting from children with school acquisition who go home and infect their parents and grandparents. It could also help to limit COVID-19-related hospitalizations at a time when other viruses are already driving pediatric hospitalizations and ICU admissions.”
Caroline Quach, the former chair of NACI and a pediatric infectious diseases expert at the University of Montreal, said that while it’s normal for parents to question vaccines for children, the trials show the vaccine is effective and less reactogenic — meaning the percentage of children aged five to 11 who received the vaccine and experienced fever, fatigue and myalgia is lower than the percentage of vaccine recipients 16 to 25 years old who experienced similar reactions.
“There were enough children in the Pfizer study to know this with confidence,” she said.
“It is brilliant that we have this vaccine for kids,” said Dr. Allison McGeer, an infectious diseases specialist and microbiologist at Mount Sinai Hospital in Toronto.
Although COVID-19 isn’t usually as serious in children as it is in older adults, she said, it is a “non-trivial disease. It’s something most parents will want their children well protected from.”
“Whatever proportion of kids get vaccinated will create a significant reduction in transmission and just let us all get back to something closer to normal,” McGeer said.
The Canadian Paediatric Society also issued a statement saying it “welcomes” the vaccine’s approval.
As part of its approval, Health Canada is requiring Pfizer-BioNTech to continue providing information to Health Canada on the safety and efficacy of the vaccine in the younger age group.
“This will provide the department with more data from ongoing studies and real-world use to ensure that the benefits of the vaccine continue to outweigh any risks, as well as to detect any potential new safety signals in any age group,” said the department.
“Health Canada and the Public Health Agency of Canada will continue to closely monitor the safety of this vaccine and will take action if any safety concerns are identified.”






Redes Sociais - Comentários