Feds sign agreements with Pfizer, Moderna for millions of doses of COVID-19 vaccines
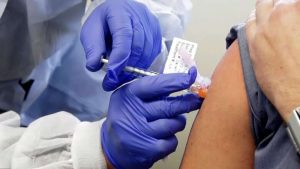
The federal government has entered two agreements to secure millions of doses of potential COVID-19 vaccines.
Public Services and Procurement Minister Anita Anand announced that Ottawa has signed deals with the pharmaceutical giant Pfizer and biotechnology firm Moderna. Pfizer will supply its BNT162 mRNA-based vaccine candidate, while Moderna will provide its mRNA-1273 vaccine candidate.
“These vaccine candidates are very promising and we all look forward to the day when restrictions can be lifted entirely,” said Anand during a news conference in Toronto along with Science and Industry Minister Navdeep Bains.
“However there is more work to do. Any potential vaccine candidate will take time to develop, properly test, mass manufacture and distribute.”
Both companies began Phase 3 clinical trials of their vaccine candidates in the last week, large-scale tests to determine how well the vaccines work.
Earlier in July both Pfizer and Moderna reported positive results from smaller trials.
The Phase 3 trials will both test the vaccines on 30,000 people, and results are expected in the fall.
All potential vaccines will require Health Canada regulatory approval prior to being used to vaccinate Canadians.
Details kept private
Public Services and Procurement Canada is also procuring the necessary equipment and supplies to manufacture and produce the vaccine in Canada, and other supplies to ensure safe immunization such as syringes, alcohol swabs and gauze.
Anand said today’s announcement marks “an important step forward.” The agreements will ensure Canadians are “at the front of the line” when a vaccine is approved.
But she wouldn’t say yet how much Canada is spending or how many doses of either vaccine candidate it will get because, she says, Canada is in talks with other domestic and international firms to secure doses of their experimental vaccines as well.
“The information we can reveal at the current time regarding doses in particular is being kept confidential because we are taking a prudent approach to the negotiations while we are engaged with other suppliers,” she said.
Vaccine development normally takes years, or decades, but scientific teams around the world are aiming to develop a vaccine for the coronavirus that causes COVID-19 in 12 to 18 months.
According to a news release, because active negotiations with other potential vaccine suppliers are underway, the government cannot disclose contract details.
The announcement comes one day after Canada’s chief public health officer Dr. Theresa Tam warned that Canadians shouldn’t expect a COVID-19 vaccine to be a “silver bullet” that will bring a swift end to the coronavirus pandemic and a return to normal.
Tam said public health officials are planning for a scenario where public health measures that have been taken so far could remain in place even after a successful vaccine is found.
Anand echoed that sentiment, urging Canadians to continue to practise physical distancing, wash their hands and wear masks in public to prevent the spread of the virus.
CBC






Redes Sociais - Comentários